Abstract
Introduction: Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is a provisional entity in the 2017 WHO classifications of the lymphoid neoplasms, as it was found that features of MEITL differed from those of enteropathy-associated T-cell lymphoma. It is now considered that MEITL is more prevalent in Asia, and almost all cases of intestinal T-cell lymphoma in Asians might be MEITL. To further elucidate the clinicopathologic features of this new disease entity, we carried out a multicenter, retrospective analysis from 9 tertiary institutes in Korea.
Patients and methods: A total of 38 patients who were diagnosed with MEITL between 2002 and 2017 from 9 institutes were included in the analysis. Medical records including age, sex, stage, presenting symptoms, laboratory findings, primary sites, and treatment outcomes were collected. The histopathologic diagnoses were centrally-reviewed by experienced lymphoma histopathologists. The primary end-point of the analysis was overall survival (OS).
Results: The median age of the patients was 59 (range, 20 - 84) and 27 patients (71.1%) were male. None of the patients had prior history of Celiac disease. Thirty one patients (81.5%) were stage I-II by the Ann-Arbor classifications, and 28 patients (73.7%) were stage I-II1&2 by the Lugano classifications. The most frequent site of involvement was jejunum (N = 20) followed by ileum (N = 17), and 11 patients had multiple site involvement. In line with the previous reports, most cases expressed CD8 (77.1%) and CD56 (92.1%), and did not expressed CD30 (5.3%), and EBER (6.9%). T-cell intracellular antigen was positive in 14 out of 17 cases (82.4%).
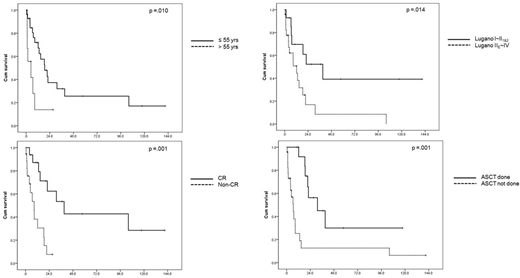
The median progression-free survival was 6.9 months (95% CI 4.2 - 9.6), and the median OS was 14.8 months (3.0 - 26.6). Thirty one patients (81.6%) received surgery, and 34 patients (89.5%) received chemotherapy. CHOP (N = 28) was the most frequently used regimen followed by CHOEP (N = 3), and ICE, IMVP-16, and EPOCH (N = 1 each). Complete response (CR) rate was 47.1%, and 14 patients had undergone autologous stem cell transplantation (ASCT). Relapse or progression was documented in 22 cases, and the most frequent site was the primary site (N = 20). Of note, relapse at central nervous system was found in 4 cases. Older age (≥ 55 years), advanced Lugano stage (IIE~IV), not achieving CR, and not receiving ASCT were associated with adverse OS.
Conclusion: Although most patients had limited stage, the clinical outcomes of MEITL patients were dismal. While the optimal management of MEITL remains undetermined, achieving CR and consolidative ASCT seem to be essential. In addition, considering the frequent local failure, as well as the CNS relapse, novel therapeutic approaches are required to improve survival.
Kim:Mundipharma: Research Funding; Merck: Research Funding; Celgene: Research Funding; Takeda: Research Funding; Roche: Honoraria, Research Funding; Kyowa-Kirin: Research Funding; Eisai: Honoraria, Research Funding; J&J: Research Funding; Celltrion: Honoraria, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.